Ifuse Implant System Reviews
Ifuse implant system reviews. These iFuse patients share their stories of how sacroiliac joint pain affected their lives and their experience with the iFuse Implant System. Keystone First Submission Date. Potential of a quicker recovery.
2 There are potential risks associated with the iFuse Implant System. Pt had chiropractic adjustments to relieve pain during which time she was informed by chiropractors that her si-joint was fused while she was out of alignment. Of a randomized trial of 148 persons with sacroiliac joint dysfunction 102 had iFuse implants and 46 had nonsurgical management.
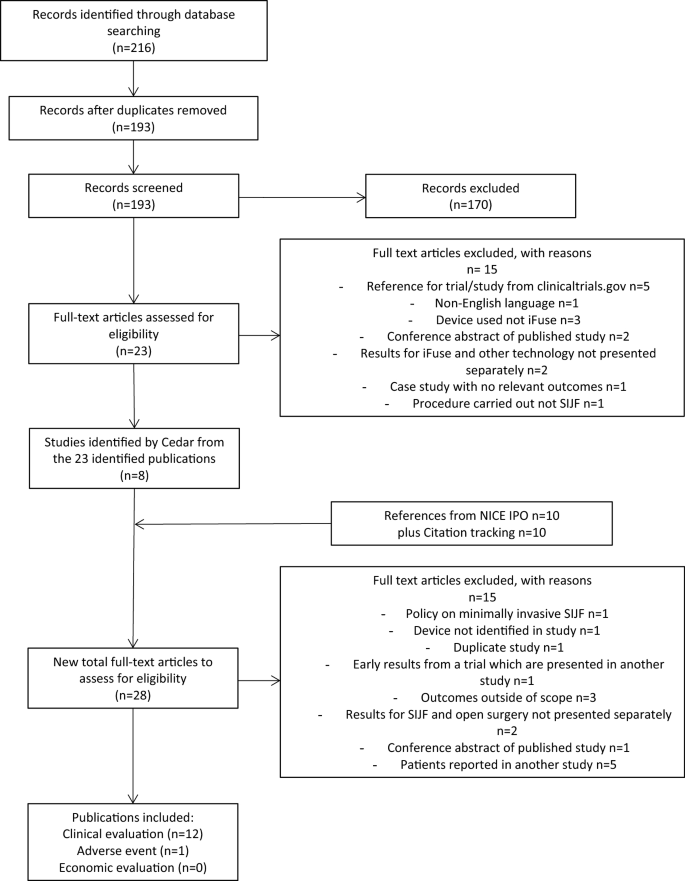
More than thirty published peer-reviewed articles demonstrate safety and effectiveness of the iFuse Implant. The iFuse procedure is a minimally invasive surgical MIS option meaning that it requires a smaller surgical incision less time in surgery about an hour and potentially a faster healing process. IFuse Published Peer-Reviewed Articles.
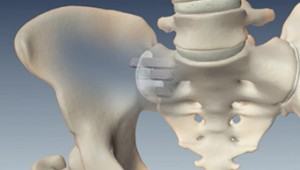
The iFuse Implant System is placed across the sacroiliac joint using minimally invasive surgery stabilising the joint and correcting any misalignment or weakness that can cause chronic pain. The iFuse Implant System procedure offers several benefits compared to traditional sacroiliac joint surgery. Six months after the procedure the iFuse group had greater pain improvement 814 versus 261 percent and disability improvement 733 versus 136 percent differences sustained at 12 months.
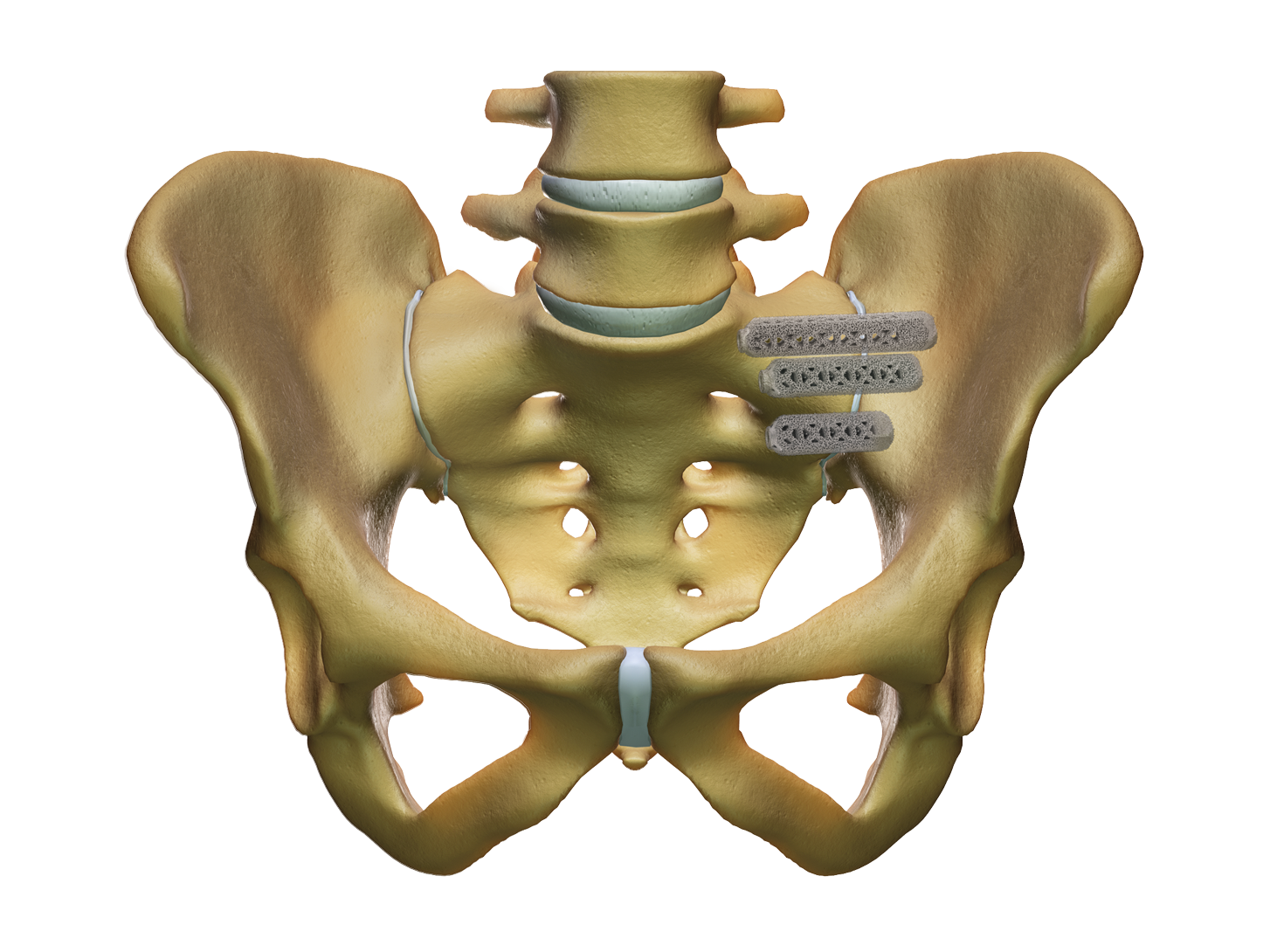
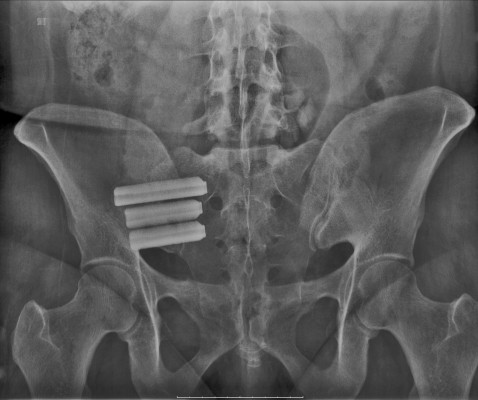
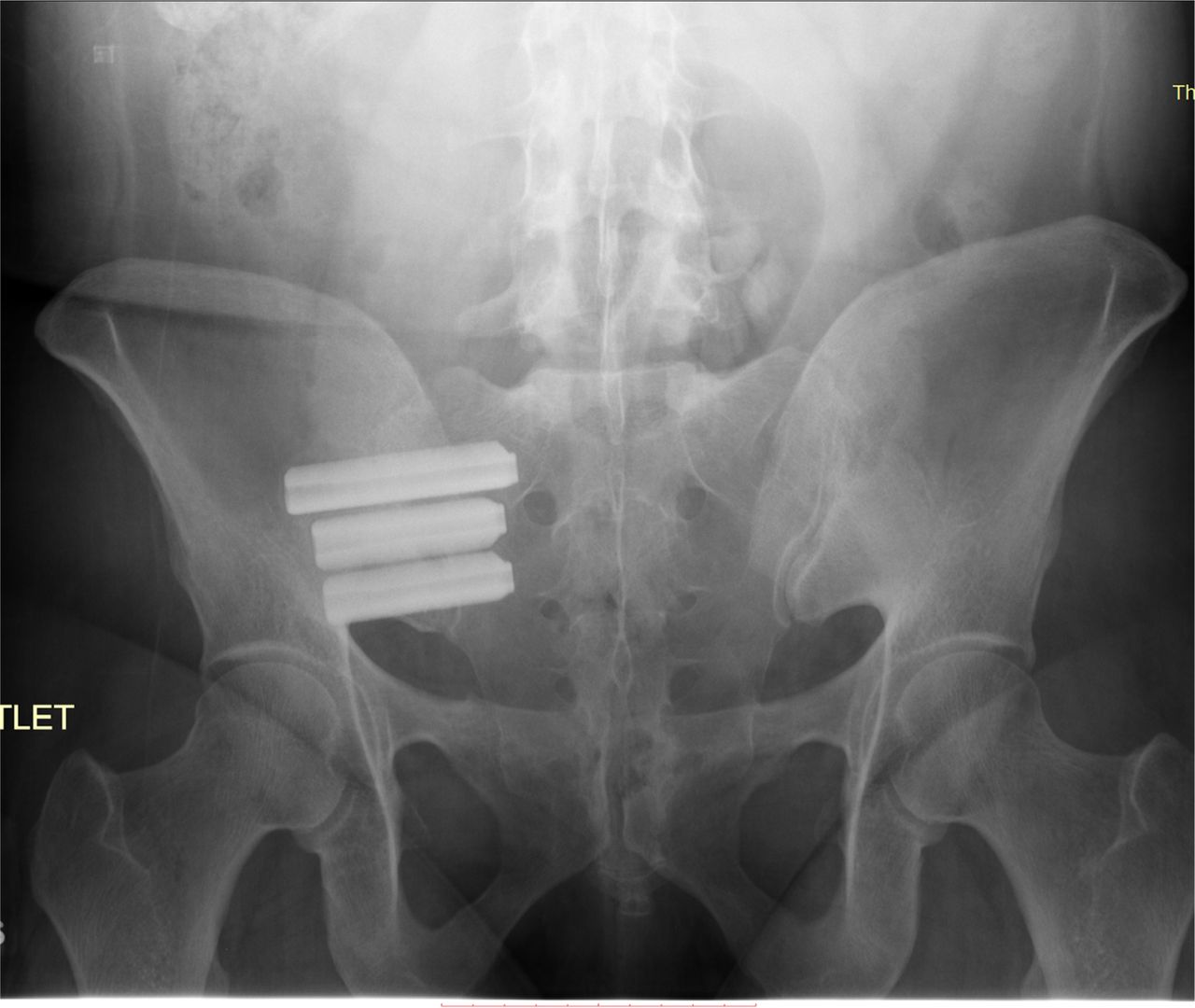
Since then more than 2500 surgeons have performed a combined total of more than 60000 SI joint fusion procedures. The iFuse procedure takes about an hour and involves three small titanium implants inserted surgically across the sacroiliac joint. Patients should discuss these risks and considerations with their physician before deciding if this treatment option is right for them.
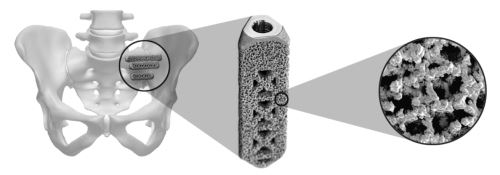
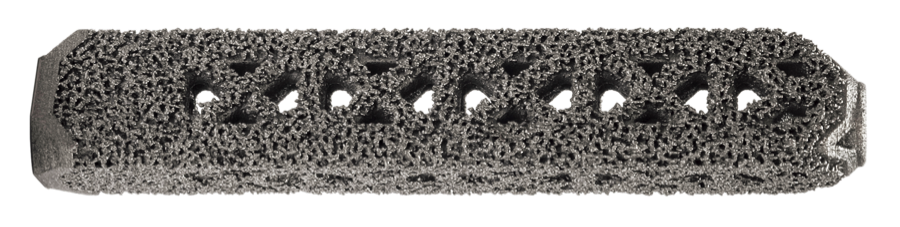
Implants are constructed from medical grade titanium alloy Ti-6Al-4V ELI per ASTM F3001 and are 3D printed with lattice regions. Physicians should review the iFuse Instructions For Use for a complete discussion of contraindications warnings precautions and risks. Porous titanium plasma spray TPS coating allows for biological fixation TPS technology used for decades in other medical applications such as orthopedics and dentistry.
The iFuse Implant System is a minimally invasive option for patients suffering from sacroiliac joint disorders including SI joint disruptions and degenerative sacroiliitis. Joint disruptions and degenerative sacroliitis.
These iFuse patients share their stories of how sacroiliac joint pain affected their lives and their experience with the iFuse Implant System.
Joint disruptions and degenerative sacroliitis. Minimal soft tissue stripping. Perspectives of iFuse Patients. 2 There are potential risks associated with the iFuse Implant System. Pt was pain free for approximately 6 months after which time the pts pain returned. Implants are constructed from medical grade titanium alloy Ti-6Al-4V ELI per ASTM F3001 and are 3D printed with lattice regions. Degeneration or disruption who underwent minimally invasive fusion using the iFuse Implant System. Pt received three ifuse implants one 7mmx55mm and two 7mmx35mm in 2010. SI-BONE is a medical device company that pioneered minimally invasive surgery of the SI joint with the iFuse Implant System.
2 There are potential risks associated with the iFuse Implant System. July 26 2019 Policy Number. Subjects were highly debilitated at baseline mean VAS pain score 78 mean ODI score 54. Pt was pain free for approximately 6 months after which time the pts pain returned. Since then more than 2500 surgeons have performed a combined total of more than 60000 SI joint fusion procedures. A separate copy of this form must accompany each policy submitted for review. The SI-BONE iFuse-TORQ Implant System consists of threaded fenestrated 3D-printed implants and associated instruments.






















.png)







Post a Comment for "Ifuse Implant System Reviews"